Vapor
From Wikipedia, the free encyclopedia
For other uses, see Vapor (disambiguation).
Water condenses into visible droplets (aerosol) after evaporating from a cup of hot tea
For example, water has a critical temperature of 374 °C (647 K), which is the highest temperature at which liquid water can exist. In the atmosphere at ordinary temperatures, therefore, gaseous water (known as water vapor) will condense into a liquid if its partial pressure is increased sufficiently.
A vapor may co-exist with a liquid (or a solid). When this is true, the two phases will be in equilibrium, and the gas-partial pressure will be equal to the equilibrium vapor pressure of the liquid (or solid).[1]
Contents
Properties
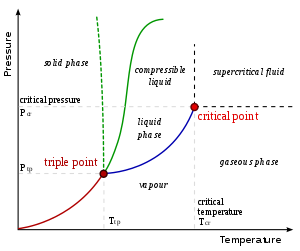
The vapor-liquid critical point in a pressure-temperature phase diagram
is at the high-temperature extreme of the liquid–gas phase boundary.
(The dotted green line gives the anomalous behaviour of water.)
Vapor is responsible for the familiar processes of cloud formation and condensation. It is commonly employed to carry out the physical processes of distillation and headspace extraction from a liquid sample prior to gas chromatography.
The constituent molecules of a vapor possess vibrational, rotational, and translational motion. These motions are considered in the kinetic theory of gases.
Vapor pressure
Main article: Vapor pressure
Liquid–vapor equilibrium
The normal boiling point of a liquid is the temperature at which the vapor pressure is equal to normal atmospheric pressure.[1]
For two-phase systems (e.g., two liquid phases), the vapor pressure of the individual phases are equal. In the absence of stronger inter-species attractions between like-like or like-unlike molecules, the vapor pressure follows Raoult's Law, which states that the partial pressure of each component is the product of the vapor pressure of the pure component and its mole fraction in the mixture. The total vapor pressure is the sum of the component partial pressures.[3]
Examples
Water vapor is responsible for humidity
- Perfumes contain chemicals that vaporize at different temperatures and at different rate in scent accords known as notes.
- Atmospheric water vapor is found near the earth's surface, and may condense into small liquid droplets and form meteorological phenomena such as fog, mist and haar.
- Mercury-vapor lamps and sodium vapor lamps produce light from atoms in excited states.
- Flammable liquids do not burn when ignited.[citation needed] It is the vapor cloud above the liquid that will burn if the vapor's concentration is between the lower flammable limit (LFL) and upper flammable limit (UFL) of the flammable liquid.
Measuring vapor
Since it is in the gas phase, the amount of vapor present is quantified by the partial pressure of the gas. Also, vapors obey the barometric formula in a gravitational field just as conventional atmospheric gases do.sumber by : https://en.wikipedia.org/wiki/Vapor



No comments:
Post a Comment